
Clinical trial info
NCT00477555
NCT02487290
ISRCTN18137204
ISRCTN87110165
Activity
We are specialized in clinical trials, management, and improving organizational efficiency
Subscribe to our mailing list
2Develop
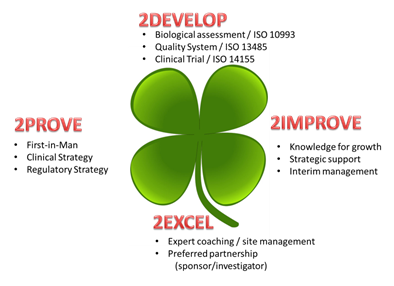
Development activities in young organizations focus on the establishment and/or improvement of the quality system (ISO 13485): an essential requirement to bring products to market.
The quality system focus on the design phase of the product, to identify the applicable ISO-standards, to perform all required pre-clinical assessments, to assure appropriate risk management (ISO 14971), to assure appropriate set up of a documentation system to establish to product master files....
FAKKEL helps you to take a fresh look at your concepts and to pinpoint critical elements of developments in view of customer and regulatory requirements.
FAKKEL helps to wrap up the required data/results in a submission dossier ready for regulatory review and get ISO 13485 certification.
FAKKEL will advise your organization to improve the performance of your clinical department and/or helps you to assess the efficiency of the CRO you are working with.


